Serious reporting inconsistencies for the bird flu vaccine trials.
More questions than answers.
I thought I’d dig into the trials which FDA cite in support of this product. I haven’t finished doing that, but I found one anomaly which looks, on its face, pretty serious.
Before describing that, it’s worth emphasising that the entirety of the “efficacy” evidence for these products rests on the finding that the products ellicit an immune response “immunogenicity” - mainly the creation of “neutralising antibodies”.
So they inject an antigen1 and show that the body creates antibodies to it. We aren’t supposed to question:
whether this provides any real immunity anyway (it didn’t for “covid”)
how blood-borne antibodies can be relevant for a respiratory infection against which the heavy lifting of the immune system takes place in the mucosal lining of the respiratory tract
what focusing on one specific antigen does to long-term immunity to that or related pathogens
whether natural immunity might be more durable and flexible than the sledgehammer approach of injecting high doses of a single antigen, and whether the injections might interfere with its development
whether the adverse events from the product as a whole offset any demonstrated benefits, since properly controlled all-cause morbidity / mortality trials sufficiently powered to answer this question are never performed
Anyway, on to the thing I found.
Trials 1 and 2 of those cited in the FDA data sheet can be found on clinicaltrials.gov2 here and here. This was a pair of jointly-run trials run around a decade ago, one in the young and one in the elderly. In each trial half the subjects received a low dose and the other half a high dose, there being no control, and subjects were followed up for a year.
The publications tab reveals a single publication from 2019 which reported on both studies.
The basic design for the 18-64 year old is summarised by this graphic.
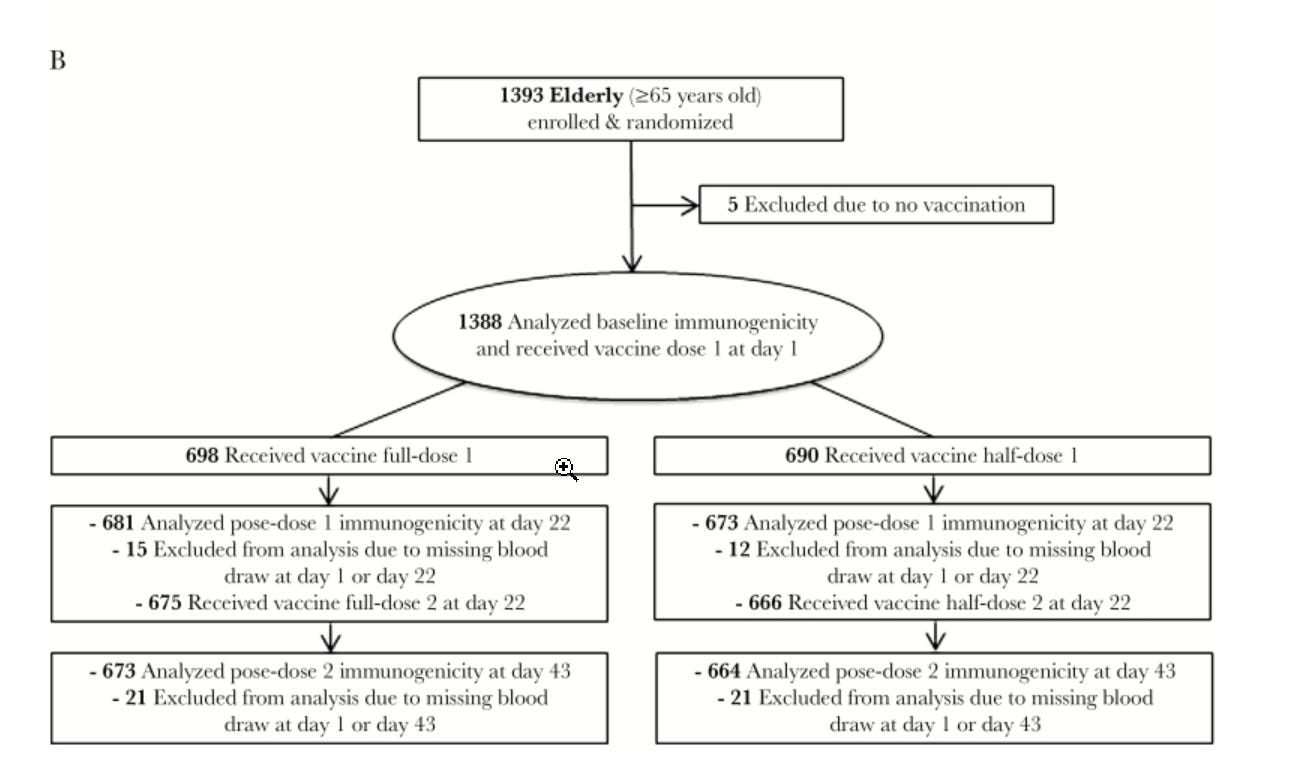
Here’s the one for the 65 years or older trial:
This is the safety summary
Between day 1 and day 43, unsolicited AEs were reported by up to 29% of subjects in the adult population and up to 32% of subjects in the elderly population, with comparable frequencies between the vaccine groups in each study. Following first or second vaccinations, 10% and 12% of subjects in the adult and elderly populations reported an unsolicited AE that was judged to be possibly or probably related to the study vaccine, respectively. Injection site bruising was the most commonly reported unsolicited AE in both age groups. At least 1 SAE was reported by 3 (<1%) subjects (appendicitis, pyelonephritis, and nerve compression) in the adult population, and by 10 (1%) subjects (atrial fibrillation, adenocarcinoma of left lung, transient ischemic attack, cholecystitis, benign positional vertigo, right inguinal hernia, acute kidney injury, infected wound of left leg, fractured ribs, and syncope) in the elderly population; none of the reported SAEs were considered to be possibly or probably vaccine-related. One elderly subject (<1%) withdrew prematurely from the study due to an unsolicited AE. Three (<1%) subjects in the adult population and 24 (2%) subjects in the elderly population were diagnosed with the new onset of chronic diseases up to day 43. Only 1 SAE had a fatal outcome (non-vaccine-related, elderly half-dose group; lung adenocarcinoma; occurred on day 155).
The rates of unsolicted AES look high to me, and would certainly warrant further examination, but what really caught my eye was the way the deaths were described.
From the above, one might be fogiven for concluding that:
one of the elderly subjects died from cancer
there were no other deaths reported
But, head over to the results section on clinicaltrials.gov.
This is what is says under “results posted”for the younger adult trial under “unsolicited AEs”
And here’s what it says for deaths in the advsere events section under the results tab on clinicaltrials.gov for the trial in elderly subjects:
ie nothing reported at all yet (which I realise is not the same as zero).
I am struggling to square these inconsistencies.
In the elderly trial, no deaths have been reported to the repository, yet one is described in the publication - but stated to be an “SAE with a fatal outcome”. Were there other deaths which weren’t reported in the publication because they weren’t classified as SAEs?
Four deaths are reported from the younger trial to the repository, but none are mentioned at all in the publications (dated 6 years after the trial commenced)?
Is it likely that there would be only one death in the elderly trial but 4 in the younger one (which had ~30% fewer subjects)?
All 4 deaths reported in the young cohort were in the higher dose cohort. Hence nearly 1% of a group of healthy subjects under 65 with average age of 39 died within a year of injection. Yet the conclusion from the publication was:
Whilst it is true that the published paper states on its face that it only presents “primary and secondary outcome data from these 13-month studies, up to study day 43”, the authors MUST have been aware of the additional deaths - assuming the data at clinicaltrials.gov was correct. And the stated objective of the study was to follow each subject “for 12 months (day 387) after the second vaccine dose to assess safety and immunogenicity”. Moreover, the one death which was reported (in the older cohort) occured at day 155.
So where is the publication reporting on the 4 deaths, as well as giving us the safety outcome data for the study in the older age group?
Does anyone else understand what has gone on here? I don’t.
As usual, it’s worth looking for potential conflicts of interest, which I reproduce below without further comment.
Update:
I emailed the author named for correspondence in the publication - a Dr Matthew Hohenboken who works at Sequiris. I have received no reply:
Obviously the mRNA products work differently - they hijack your body’s cells and instruct them to make the antigen (or so they say).










Did you find anything on murder of the unborn? I believe Naomi Wolf's team saw from the Pfizer covid documents that miscarriages in their trials were considered 'resolved medical issues.'
I will be shocked if anyone will take it...but I've been shocked before.